Abstract
Objectives
Previous literature showed that the diagnostic accuracy of computed tomographic angiography (CTA) is not equally comparable with that of the rarely used golden standard of digital subtraction angiography (DSA) for detecting blunt cerebrovascular injuries (BCVI) in trauma patients. However, advances in CTA technology may prove CTA to become equally accurate. This study investigated the diagnostic accuracy of CTA in detecting BCVI in comparison with DSA in trauma patients.
Methods
An electronic database search was performed in PubMed, EMBASE, and Cochrane Library. Summary estimates of sensitivity, specificity, positive and negative likelihood, diagnostic odds ratio, and 95% confidence intervals were determined using a bivariate random-effects model.
Results
Of the 3293 studies identified, 9 met the inclusion criteria. Pooled sensitivity was 64% (95% CI, 53–74%) and specificity 95% (95% CI, 87–99%) The estimated positive likelihood ratio was 11.8 (95%, 5.6–24.9), with a negative likelihood ratio of 0.38 (95%, 0.30–0.49) and a diagnostic odds ratio of 31 (95%, 17–56).
Conclusion
CTA has reasonable specificity but low sensitivity when compared to DSA in diagnosing any BCVI. An increase in channels to 64 slices did not yield better sensitivity. There is a risk for underdiagnosis of BCVI when only using DSA to confirm CTA-positive cases, especially in those patients with low-grade injuries.
Key Points
• Low sensitivity and high specificity were seen in identifying BCVI with CTA as compared to DSA.
• Increased CTA detector channels (≤ 64) did not lead to higher sensitivity when detecting BCVI.
• The use of CTA instead of DSA may lead to underdiagnosis and, consequently, undertreatment of BCVI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blunt cerebrovascular injuries (BCVI) collectively describe all non-penetrating traumatic injuries to the extra- or intracranial carotid and vertebral arteries. The mechanism of injury is either high-energy flexion, extension, or rotation of the neck, or a direct blow to or laceration of the blood vessels. At the level of the vessel wall, there is a risk of tear formation of the tunica intima because of the increased arterial strain. Expedited by a trauma-induced state of hypercoagulability caused by the initial trauma, the exposed subendothelial collagen activates the coagulation cascade, leading to an intraluminal thrombus formation at the site of the tear or a complete vessel occlusion. The arterial defect can also be a gateway for blood to enter the underlying layers of the vessel wall and can cause the formation of a traumatic (pseudo) aneurysm. As a result, patients with a BCVI are at risk of a secondary brain injury caused either by thromboembolism or occlusion of the artery [1,2,3,4].
Previously, BCVI was considered a rare cause of cerebral ischemia and ischemic stroke. Due to improved diagnostic imaging modalities, awareness, and the introduction of standard screening protocols, such as the Memphis and (modified) Denver criteria, the reported incidence of BCVI among blunt trauma patients has increased over recent years [5,6,7,8,9]. The prevalence ranges from 1–2% in patients with blunt trauma to 9% in patients with a severe head injury [3, 10, 11]. When comparing BCVI to non-traumatic brain injuries such as stroke, BCVI is associated with poorer cognitive outcomes, although long-term outcomes following BCVI are missing [12].
There is a 72-h window after injury to provide anti-aggregation and anticoagulation therapy to reduce the risk of secondary brain injury [4]. Screening of patients suspected of BCVI remains pivotal as up to 80% of these patients do not display neurological symptoms at presentation [13, 14]. The golden standard for diagnosing BCVI is digital subtraction angiography (DSA). However, non-invasive and fast screening modalities such as computed tomography angiography (CTA) are increasingly utilized in the acute phase [15,16,17,18]. A previously published meta-analysis showed great variability in the sensitivity of BCVI detection using CTA when compared to DSA [19]. Although DSA is the golden standard to date, recent data suggest that CTA with 64 channels has comparable sensitivity rates in diagnosing BCVI and could potentially replace DSA [19, 20]. Therefore, we performed a systematic review and meta-analysis to evaluate the contribution of new data on CTA sensitivity in diagnosing BCVI [19]. We hypothesized that CTA would result in similar accuracy for diagnosing BCVI compared to the golden standard DSA.
Material and methods
Literature search
Studies containing CTA as diagnostic imaging for BCVI that were published until February 24, 2021, were screened independently by two investigators (C.C.K., W.B.S.). An electronic database search was performed in both PubMed, EMBASE, and Cochrane Library using the following keywords: carotid, carotid artery, vertebral artery, intracranial, extracranial, neck, vertebral and vascular system, combined with blunt wound, or blunt trauma, or nonpenetrating injury/wound using the Boolean operator AND for the population. The index and reference test were defined using the keywords: computed tomography angiography, CTA, angiography, and angiotomography, digital subtraction angiography, digital subtraction arteriography, DSA, cerebral angiography, and diagnosis. Additional publications were identified through citation chaining of the bibliography of reviews and other potentially relevant studies.
Study selection and data extraction
Both investigators (C.C.K., W.B.S.) reviewed titles and abstracts for relevance and identified potentially relevant citations for full-text review using the online reviewing tool Rayyan (http://rayyan.qcri.org) [21]. The complete inclusion and exclusion criteria are shown in Table 1.
Investigators extracted the subsequent data: study design; date of patient screening; study location; inclusion and exclusion criteria; number of patients included; number of patients excluded; number of patients included in the final analysis; reference and index test; mean age and gender of participants; primary unit of analysis; who reviewed the reference and index test and whether this was done blinded; the arteries examined for BCVI; the type of CT scanner and DSA equipment; the number of slices; slice thickness; size interval; level of reconstruction; injection rate; type of contrast used in CTA and DSA; typical contrast volume used in CTA and DSA; true positives, negatives, false positives, and negatives.
Assessment of methodological quality
Methodological quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies, second version (QUADAS-2), which investigates both risks of bias as well as applicability concerns [22]. QUADAS-2 uses 7 questions to assess study selection and setting, conduct, and interpretation of the reference and index test, and flow and timing using three levels of bias (high, low, and unclear). Patient selection was considered at low risk of bias when a consecutive or random sample of patients was enrolled, when the case-control design was avoided, and when a study avoided inappropriate exclusions. A low-risk setting was one in which all patients underwent both CTA and DSA. Additionally, there was a low risk of bias in the interpretation of the index and/or reference test if the reviewers had no prior knowledge of the results of the reference test or the index test. A maximum of 48-h interval between CTA and DSA is presumed to be appropriate [19].
Data synthesis
Some studies reported data on blunt carotid artery injury (BCVIcarotid) or blunt vertebral artery injury (BCVIvertebral) separately but did not include data on BCVI per patient [6, 19, 23,24,25]. Patients diagnosed with BCVI could potentially have multiple injuries to either or both the carotid and vertebral arteries. Therefore, studies that only reported results for BCVIcarotid and BCVIvertebral did not allow for calculation of diagnostic accuracy per patient. Instead, all data were combined in one larger overall group called “any BCVI.” In this group, true and false positives and negatives were either assessed per patient and/or from the combined results of BCVIcarotid and BCVIvertebral if no per-patient data was given. Separate analyses on diagnostic accuracy were also performed for BCVIcarotid and BCVIvertebral .
When true and false positive and negative findings were separately reported by different radiologists, the average of each observation was calculated for that study population. Additionally, and if reported separately, the sum of the common, cervical, or intracranial carotid artery was calculated to determine BCVIcarotid. Likewise, the sum of the cervical or intradural vertebral artery was calculated to determine BCVIvertebral.
Statistical analysis
True and false positives and negatives were used to individually calculate the sensitivity and specificity of CTA for each study. The 95% confidence interval was calculated using the Clopper-Pearson interval method [26]. Summary estimates of sensitivity, specificity, positive and negative likelihood, and diagnostic odds ratio and their 95% confidence intervals were determined for CTA in BCVIcarotid, BCVIvertebral, and any BCVI using a bivariate random-effects model. This allowed the heterogeneity beyond chance between studies to be considered. The percentage of total variation across studies was evaluated using forest plots, chi-square, Cochrane Q, and I2, which measures the impact of unobserved heterogeneity [27]. We considered I2 values between 0 and 50% as medium heterogeneity, while values > 50% were considered high heterogeneity, and p values < 0.05 significant.
Hierarchical summary receiver operating characteristic (SROC) plots were created for visual assessment of the threshold effect by calculating the squared correlation coefficient estimate from the between-study covariance parameter [28]. Within the SROC plot, observed data points, a summary operating point of sensitivity and specificity, and 95% confidence interval contour were displayed.
Cook’s distance was determined to analyze the influence of each study [29]. Outliers were evaluated using scatter plots using standardized predicted random effects and bivariate box plots [30]. Publication bias was assessed using Deek’s funnel plot asymmetry (p < 0.10 indicating significant asymmetry) [31]. To explore sources of heterogeneity, subgroup analyses, and univariate meta-regression was used. All analyses, except subgroup analyses and univariate meta-regression, were performed using STATA 16.0 (StataCorp. 2019. Statistical Software: Release 16: StataCorp LLC.) in combination with the MIDAS command [32]. Subgroup analyses and meta-regression were performed using Open Meta Analyst [33].
Results
Study selection and inclusion
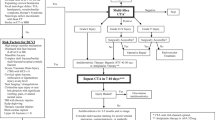
Our electronic database search of PubMed, EMBASE, and the Cochrane Library yielded 3293 studies, of which 92 studies were identified as duplicates. Of the 3204 studies screened for title and abstract, 102 articles were found eligible for full-text assessment. subsequently, 93 articles were excluded for various reasons, including not having DSA as a reference test for any and/or all patients (29 studies), not reporting the outcome or population of interest (21 studies), no CTA as an index test for any and or all patients (8 studies), non-original studies (14 review studies), wrong study design (14 studies), and no full-text availability (7 studies). Finally, 9 studies were included for quality assessment (Fig. 1) [6, 20, 23,24,25, 34,35,36,37].
Quality assessment
The risk of bias and applicability were assessed using QUADAS-2 tool questions for all included (Fig. 2). Two studies reported no or unclear data on whether patients were consecutively enrolled or randomly selected, or whether a case-control design was avoided [25, 34]. One study systematically applied CTA in patients suspected of BCVI and used DSA in a minority of those patients [23]. They, therefore, anticipated a higher rate of positives in their study population. We also anticipated high risk of bias in patient selection for this study. Three studies clearly stated whether the results of the index test were interpreted without prior knowledge of the results of the reference test [20, 24, 25]. This was also the case for four studies regarding the reference test [20, 24, 25, 35]. In two studies, it was unclear whether all patients were included in the analysis [35, 37]. Overall, there were no concerns for applicability with either of the studies included. The risk of bias was not considered great enough to exclude any study from further analysis.
Study characteristics
All study and patient characteristics are summarized in Table 2. Of the 9 included studies, 3 were conducted retrospectively, 5 prospectively, and one both retrospectively and prospectively. A total of 1918 patients were screened for BCVI with both DSA and CTA, 67% male with an overall mean age of 40.5 years (range 1–94).
Six studies used CTA with 16 slices or more [20, 24, 25, 35,36,37]. One study reported both data for 16 and 64 slices CTA [36]. We chose to consider these as two separate data sets in our analyses. Most studies used CTA with Omnipaque (Amersham Health Inc.), with varying concentrations between 300 and 350 mg/mL [35,36,37]. DSA was used as a reference test in all studies, with descriptions on model and contrast type being noted in almost all except for two studies [6, 34]. A complete summary of the geographical information for all commercial products is shown in Appendix 1.
All diagnostic accuracy estimates are listed in Table 3. Overall, three studies reported both outcomes for BCVI per-patient and per-artery [20, 35, 37]. The per-patient data were used to calculate true and false positives and negatives in the category “any BCVI.” If unavailable, the sum of both carotid and vertebral injuries was used.
Outlier detection, influence analysis, and publication bias
Using standardized predicted random effects and bivariate box plots, outliers were identified for all four categories (per-patient, BCVIcarotid, BCVIvertebral, and any BCVI). In one study, outliers were detected in the category “any BCVI” [35]. Additionally, Cook’s distance depicted the same study as an outlier.
Publication bias was assessed using Deek’s funnel plot asymmetry test and visual funnel plot analysis. There was no significant asymmetry between studies in the four different categories, with p values ranging between 0.187 for BCVIcarotid and 0.914 for any BCVI (Table 6). No concern for publication bias was found.
Pooled diagnostic accuracy estimates for CTA versus DSA for any form of BCVI
After outlier removal, 8 studies were included in the analysis for diagnostic accuracy estimates of CTA versus DSA for any BCVI [6, 20, 23,24,25, 34, 36, 37]. Figure 3 shows the SROC plot for the detection of any BCVI with CTA vs DSA and the covariation in sensitivity and specificity of the studies included. Pooled sensitivity was 64% (95% CI, 53–74%) and specificity 95% (95% CI, 87–99%) (Figs. 4 and 5). There was a high degree of heterogeneity between studies, with an I2 for sensitivity of 76.07 (p < 0.01) and specificity of 95.20 (p < 0.01) (Fig. 4). Forest plots for the pooled diagnostic odds ratio and positive and negative likelihood ratio are provided in Appendix 2.
Pooled diagnostic accuracy estimates for CTA versus DSA in BCVIcarotid and BCVIvertebral
To determine diagnostic accuracy for CTA vs DSA in BCVIcarotid and BCVIvertebral, data were combined from 7 studies [6, 20, 23,24,25, 35, 37]. Pooled sensitivity was 70% (95% CI, 52–84%) and specificity 98% (95% CI, 94–99%) in BCVIcarotid (Table 3). Pooled sensitivity was 70% (95% CI, 55–82%) and specificity 99% (95% CI, 94–100%) in BCVIvertebral (Table 3).
There was a high degree of heterogeneity between studies, with an I2 for sensitivity of 86.87 (p < 0.01) and specificity of 97.64 (p < 0.01) for BCVIcarotid and an I2 for sensitivity of 76.07 (p < 0.01) and specificity of 95.20 (p < 0.01) in BCVIvertebral.
Subgroup analyses and meta-regression
Exploration of the sources of heterogeneity found there was no significant difference between studies based on publication year and the number of CT detector rows when calculating combined pooled sensitivity and specificity of CTA for the detection of any BCVI (Table 4). There was a significant difference, however, when comparing per-artery and per-patient studies. Sensitivity was reported at 70.3% (95% CI, 41.3–88.9) in ≥ 16-slice CTA vs 63.1% (95% CI, 46.3–77.2) in < 16-slice CTA (p = 0.868), and specificity at 96.1 (95% CI, 86.5–98.9) in ≥ 16-slice CTA vs 94.6 (95% CI, 61.–99.5) in < 16-slice CTA (p = 0.683).
Discussion
This systematic review and meta-analysis showed low sensitivity and moderate to good specificity for CTA (Table 5) in diagnosing BCVI as defined by DSA (Table 6). This is in line with previously published results on the diagnostic accuracy between CTA and DSA for low-channel CTA. However, contrary to our hypothesis and previous results by Paules et al, this study did not find that an increase of CTA channels beyond 16 slices showed higher diagnostic accuracy in detecting BCVI. A possible explanation for the observed low sensitivity is the absence of recent studies including both higher channel CTA (i.e., > 64 slices) and DSA as a reference test [38,39,40]. This could be attributed to the invasiveness of DSA and the possible complications associated with the technique, higher cost, and its availability in both equipment and expertise. Although one might expect a higher diagnostic accuracy with high-channel CTA, its intra- and interobserver reliability and the expected better yield of detection of BCVI are not established.
Due to its widespread availability, lower invasiveness, and cost-effectiveness, CTA is already widely used in clinical practice to detect BCVI. The complex hemodynamic nature of BCVI, the applied protocols, and guidelines have mainly focused to rule out any patients with a false negative finding for BCVI with CTA, thus eliminating the need for anticoagulation treatment. Although DSA is still presumed to be the golden standard, it is selectively and mostly used to confirm the presence of BCVI in either clinically suspected cerebrovascular injury with negative CTA or to better visualize a highly suspected BCVI on DSA. Therefore, the possibility and desirability of replacing DSA for CTA as the golden standard in diagnosing BCVI is still a subject of discussion.
The use of DSA to only confirm the presence of BCVI on CTA raises other concerns. Studies have shown that neurological symptoms in patients with BCVI might be delayed [12, 13]. Due to the low sensitivity of CTA shown in this study, a substantial number of patients with a false-negative outcome for BCVI are missed, which would leave them untreated. Hence, the pivot question seems to be how harmful undertreatment is in undiagnosed BCVI patients. Assuming that high-grade BCVIs are detected on both DSA and CTA imaging, patients with low-grade BCVI (such as intimal irregularity or intramural hematoma) would be most at risk for undertreatment. However, despite the available recommendations to treat this patient category either with aspirin or heparin for secondary prevention of thrombus formation [39, 41, 42], there is no established evidence that this has a beneficial effect in preventing cerebrovascular events.
This study has some important limitations. First, due to the heterogenic nature of the population in different studies and the difference in sample size, it is difficult to extrapolate the finding to the general trauma population. The use of different equipment with different settings and contrasts limits the applicability of the results to all kinds of equipment used in the field, especially when outdated CT scanners are increasingly being replaced by scanners with 256-detector rows, low-kVp imaging, multi-energy reconstruction, and all kinds of post-processing 3D reconstruction technology. Also, nontrivial settings such as the velocity of injection of the contrast might have influenced the diagnosis of BCVI. Future studies should therefore focus on more modern scanners and standardized protocols.
Second, the human factor is even more important in visualizing subtle changes in imaging. There is no evidence on how accurate radiologists or other specialists are in diagnosing (mild) BCVI in CTA. Subsequently, factors such as special interest, experience, exposure, and central referral might influence the accuracy to visualize even small and subtle changes in imaging [43]. This should be the focus of future studies to rule out the bias introduced by human inconsistency and establish minimum requirements in caregivers to establish a reliable and reproducible statement.
Third, there is a risk of selection bias when including only patients diagnosed with both CTA and DSA. It is possible that, due to time restrictions, patients with polytrauma suspected for BCVI were not included for both CTA and DSA screening. This could lead to an underrepresentation of patients with high-grade BCVI or even those with low-grade dissections that are only screened when severe and/or delayed neurological symptoms occur.
In conclusion, this systematic review and meta-analysis showed moderate to good specificity but low sensitivity of CTA in diagnosing BCVI compared to DSA. Furthermore, CTA with higher channels (16-64) did not increase the diagnostic accuracy of CTA compared to lower channels (<16). This might lead to a risk of undertreatment of BCVI in false-negative cases, especially in those with low-grade injuries. It is unclear whether this is associated with an increased risk of cerebrovascular events. Future studies should focus on a. the diagnostic accuracy of nowadays widely available 256-channel CTA, the inter- and intra-observer reliability, and on the harmfulness of undertreatment of BCVI patients.
Abbreviations
- BCVI:
-
Blunt cerebrovascular injury
- CTA:
-
Computed tomography angiography
- DSA:
-
Digital subtraction angiography
References
Debette S, Leys D (2009) Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol 8:668–678
Rutman AM, Vranic JE, Mossa-Basha M (2018) Imaging and management of blunt cerebrovascular injury. Radiographics 38:542–563
Esnault P, Cardinale M, Boret H et al (2017) Blunt cerebrovascular injuries in severe traumatic brain injury: Incidence, risk factors, and evolution. J Neurosurg 127:16–22
Harrigan MR (2020) Ischemic stroke due to blunt traumatic cerebrovascular injury. Stroke 51:353–360
Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Burch JM (1999) Blunt carotid arterial injuries: implications of a new grading scale. J Trauma Acute Care Surg 47:845
Miller PR, Fabian TC, Croce MA et al (2002) Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg 236:386–393 discussion 393-385
Ciapetti M, Circelli A, Zagli G et al (2010) Diagnosis of carotid arterial injury in major trauma using a modification of Memphis criteria. Scand J Trauma Resusc Emerg Med 18:61
Jacobson LE, Ziemba-Davis M, Herrera AJ (2015) The limitations of using risk factors to screen for blunt cerebrovascular injuries: the harder you look, the more you find. World J Emergency Surg 10:46
Fleck SK, Langner S, Baldauf J, Kirsch M, Kohlmann T, Schroeder HW (2011) Incidence of blunt craniocervical artery injuries: use of whole-body computed tomography trauma imaging with adapted computed tomography angiography. Neurosurgery 69:615–623 discussion 623-614
Franz RW, Willette PA, Wood MJ, Wright ML, Hartman JF (2012) A systematic review and meta-analysis of diagnostic screening criteria for blunt cerebrovascular injuries. J Am Coll Surg 214:313–327
Hundersmarck D, Slooff W-BM, Homans JF et al (2019) Blunt cerebrovascular injury: incidence and long-term follow-up. Eur J Trauma Emergency Surg:1–10
DiCocco JM, Fabian TC, Emmett KP et al (2013) Functional outcomes following blunt cerebrovascular injury. J Trauma Acute Care Surg 74:955–960
Biffl WL, Moore EE, Burch JM (2002) Diagnosis and management of thoracic and abdominal vascular injuries. Trauma 4:105–115
Burlew CC, Biffl WL (2011) Imaging for blunt carotid and vertebral artery injuries. Surg Clin North Am 91:217–231
Cothren CC, Moore EE, Ray CE Jr et al (2005) Screening for blunt cerebrovascular injuries is cost-effective. Am J Surg 190:849–854
Kaye D, Brasel KJ, Neideen T, Weigelt JA (2011) Screening for blunt cerebrovascular injuries is cost-effective. J Trauma Acute Care Surg 70:1051–1057
Malhotra A, Wu X, Kalra VB, Schindler J, Matouk CC, Forman HP (2016) Evaluation for blunt cerebrovascular injury: review of the literature and a cost-effectiveness analysis. AJNR Am J Neuroradiol 37:330–335
Brommeland T, Helseth E, Aarhus M et al (2018) Best practice guidelines for blunt cerebrovascular injury (BCVI). Scand J Trauma Resusc Emerg Med 26:90
Roberts D, Chaubey V, Zygun D et al (2012) Diagnostic accuracy of computed tomographic angiography for blunt cerebrovascular injury detection in trauma patients: a systematic review and meta-analysis. Critical Care Med 40:177
Paulus EM, Fabian TC, Savage SA et al (2014) Blunt cerebrovascular injury screening with 64-channel multidetector computed tomography: more slices finally cut it. J Trauma Acute Care Surg 76:279–283 discussion 284-275
Mourad Ouzzani HH, Fedorowicz Z, Elmagarmid A (2016) Rayyan — a web and mobile app for systematic reviews. Systematic Rev 5:210
Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Bub LD, Hollingworth W, Jarvik JG, Hallam DK (2005) Screening for blunt cerebrovascular injury: evaluating the accuracy of multidetector computed tomographic angiography. J Trauma 59:691–697
DiCocco JM, Emmett KP, Fabian TC, Zarzaur BL, Williams JS, Croce MA (2011) Blunt cerebrovascular injury screening with 32-channel multidetector computed tomography: more slices still don't cut it. Ann Surg 253:444–450
Sliker CW, Shanmuganathan K, Mirvis SE (2008) Diagnosis of blunt cerebrovascular injuries with 16-MDCT: accuracy of whole-body MDCT compared with neck MDCT angiography. AJR Am J Roentgenol 190:790–799
Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404–413
Higgins J, Thompson S, Deeks J, Altman D (2002) Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy 7:51–61
Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM (2008) Systematic reviews of diagnostic test accuracy. Ann Intern Med 149:889–897
Muller KE, Mok MC (1997) The distribution of cook's d statistic. Commun Stat Theory Methods 26(3). https://doi.org/10.1080/03610927708831932
Rousseeuw PJ, Ruts I, Tukey JW (1999) The Bagplot: A Bivariate Boxplot, The American Statistician, 53(4):382–387. https://doi.org/10.1080/00031305.1999.10474494
Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58:882–893
Dwamena B (2007) MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies. Available via https://ideas.repec.org/c/boc/bocode/s456880.html
Wallace BC, Schmid CH, Lau J, Trikalinos TA (2009) Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol 9:80
Biffl WL, Ray CE Jr, Moore EE, Mestek M, Johnson JL, Burch JM (2002) Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma 53:850–856
Eastman AL, Chason DP, Perez CL, McAnulty AL, Minei JP (2006) Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma 60:925–929 discussion 929
Goodwin RB, Beery PR 2nd, Dorbish RJ et al (2009) Computed tomographic angiography versus conventional angiography for the diagnosis of blunt cerebrovascular injury in trauma patients. J Trauma 67:1046–1050
Malhotra AK, Camacho M, Ivatury RR et al (2007) Computed tomographic angiography for the diagnosis of blunt carotid/vertebral artery injury: a note of caution. Ann Surg 246:632–642 discussion 642-633
Emmett KP, Fabian TC, DiCocco JM, Zarzaur BL, Croce MA (2011) Improving the screening criteria for blunt cerebrovascular injury: the appropriate role for computed tomography angiography. J Trauma 70:1058–1063 discussion 1063-1055
Shahan CP, Magnotti LJ, McBeth PB, Weinberg JA, Croce MA, Fabian TC (2016) Early antithrombotic therapy is safe and effective in patients with blunt cerebrovascular injury and solid organ injury or traumatic brain injury. J Trauma Acute Care Surg 81:173–177
Wang AC, Charters MA, Thawani JP, Than KD, Sullivan SE, Graziano GP (2012) Evaluating the use and utility of noninvasive angiography in diagnosing traumatic blunt cerebrovascular injury. J Trauma Acute Care Surg 72:1601–1610
Bromberg WJ, Collier BC, Diebel LN et al (2010) Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma and Acute Care Surg 68:471–477
Grandhi R, Weiner GM, Agarwal N et al (2018) Limitations of multidetector computed tomography angiography for the diagnosis of blunt cerebrovascular injury. J Neurosurg 128:1642–1647
Liang T, Tso DK, Chiu RY, Nicolaou S (2013) Imaging of blunt vascular neck injuries: a review of screening and imaging modalities. AJR Am J Roentgenol 201:884–892
Acknowledgements
The authors would like to acknowledge dr. Paco M. J. Welsing, associate Professor Clinical Epidemiology at University Medical Center Utrecht, for his methodological assistance.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is W. B. M. Slooff.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
dr. Paco M. J. Welsing, associate Professor of Clinical Epidemiology at University Medical Center Utrecht kindly provided statistical advice for this manuscript.
Informed consent
Not applicable
Ethical approval
Institutional Review Board approval was not required because of the nature of this review.
Methodology
-
retrospective
-
diagnostic or prognostic study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kik, C.C., Slooff, WB.M., Moayeri, N. et al. Diagnostic accuracy of computed tomography angiography (CTA) for diagnosing blunt cerebrovascular injury in trauma patients: a systematic review and meta-analysis. Eur Radiol 32, 2727–2738 (2022). https://doi.org/10.1007/s00330-021-08379-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08379-7